-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Barausse, Vittoria Correale, Aleksia Curkovic, Licia Finotto, Emilio Riginella, Eleonora Visentin, Carlotta Mazzoldi, The role of fisheries and the environment in driving the decline of elasmobranchs in the northern Adriatic Sea, ICES Journal of Marine Science, Volume 71, Issue 7, September/October 2014, Pages 1593–1603, https://doi.org/10.1093/icesjms/fst222
Close - Share Icon Share
Abstract
Elasmobranch populations are declining worldwide, calling for urgent assessment of fishery exploitation and application of effective conservation strategies. Here, we applied a novel approach, integrating long-term time-series of landings (1945–2012) and extensive surveys at the fish market of Chioggia, Italy, home of the major fishing fleet of the northern Adriatic Sea, to evaluate the status of elasmobranch populations and fisheries in the one of the most fished Mediterranean basins. The time-series highlight a dramatic decline in elasmobranch landings, particularly for skates and catsharks (Scyliorhinus spp.), whose current catch rates are 2.4 and 10.6% of the average 1940s levels, respectively. These data likely reflect similar large reductions in abundance, as indicated by the analysis of catch-per unit-effort time-series. The biomass of landed skates and catsharks showed regular fluctuations that disappeared after the collapse of the landings. Elasmobranch market composition, assessed through the sampling of 11 900 specimens from 2006 to 2013, included 14 species, but was dominated by just two: Mustelus mustelus and M. punctulatus, which represented more than 60% of the catch. The proportion of sexually immature individuals was generally very high, up to 83% of landed females and 71% of landed males, depending on the species. Although some correlations were detected between landings and local hydrography or climatic indices, the analyses of landings and surveys at the fish market identified fishery exploitation as the main driver of the striking, long-term elasmobranch decline in the northern Adriatic Sea, calling for urgent management actions to improve the conservation status of these fish.
Introduction
Elasmobranchs are exploited worldwide, either as the target of specific fisheries or, more often, as bycatch of fisheries targeting other more abundant or valuable resources (Walker, 1998; Stevens et al., 2000). As bycatch, elasmobranchs are not subject to economic extinction when fishing drives them to low densities; consequently, some elasmobranch species have already become locally extinct (Casey and Myers, 1998; Musick et al., 2000). Moreover, although data on elasmobranch landings and stock status are often poor or non-existent (Polidoro et al., 2008; Worm et al., 2013), the emerging picture shows a dramatic decline or collapse of several elasmobranch populations, particularly the large predatory species (Stevens et al., 2000; Myers and Worm, 2005; Dulvy et al., 2008; Worm et al., 2013). On the IUCN Red List, 16% of the evaluated species of sharks and their relatives (n = 1090) are considered at higher risk of extinction, and only 25% are evaluated to be of least concern for conservation, while evaluation data are deficient for more than 46% (www.iucnredlist.org, last accessed 6 May 2013). Life history characteristics, such as large size, slow growth rate, late maturity, and low fecundity result in low reproductive rates and make elasmobranchs more sensitive to exploitation than bony fish (Dulvy and Reynolds, 2002; Field et al., 2009). These attributes make elasmobranchs inadequately resilient to fishing mortality, inclined to rapid stock depletion, and unable to quickly rebound from population reductions (Smith et al., 1998; Stevens et al., 2000; Myers and Worm, 2005; Dulvy et al., 2008).
Worldwide, elasmobranch fisheries have expanded in response to growing demand (particularly for highly valuable parts such as shark fins), the accessibility of new areas (i.e. open ocean, deep-sea bottom), and the utilization of highly technically equipped fishing vessels (Casey and Myers, 1998; Clarke et al., 2007; Polidoro et al., 2008; Worm et al., 2013). These developments, together with the decline in several elasmobranch stocks, have led to a call for an improvement in international actions for the management of sharks and related species to ensure sustainable elasmobranch fisheries (FAO, 2000; Lucifora et al., 2011; Bradai et al., 2012).
The Mediterranean Sea represents a hotspot of marine biodiversity that is exposed to multiple threats, including fishing pressure, habitat loss and degradation, pollution, eutrophication, and, more recently, climate change and invasion by alien species (Coll et al., 2010). Here, elasmobranchs are represented by ∼85 shark and batoid species (Bradai et al., 2012) and have been highly exploited, with more than 40% of the species evaluated under threat (Cavanagh and Gibson, 2007; Bradai et al., 2012). A marked decline has been highlighted, not only for large species (Ferretti et al., 2008) but also for smaller commercially important species in different Mediterranean areas (Dell'Apa et al., 2012; Ferretti et al., 2013; Ligas et al., 2013). Within the Mediterranean Sea, the Adriatic Sea (particularly its northern part) represents a productive, heavily exploited subbasin (Barausse et al., 2009) whose marine communities are strongly influenced by anthropogenic pressures (e.g. fishery and nutrient inputs) and environmental factors (Barausse et al., 2011). In the northern Adriatic Sea, the status of elasmobranch communities has been investigated using scientific surveys, which have highlighted the decrease of several species, of which 11 have almost completely disappeared (Ferretti et al., 2013). These data have the advantage of being standardized and species-specific, but they are clearly limited in time and sampling effort. In contrast, fishery statistics often report elasmobranchs aggregated into multispecies categories, are not standardized and suffer from an unreported quota of landings (Walker, 1998; Dulvy et al., 2000; Myers and Worm, 2005; Worm et al., 2013). However, they provide an extensive picture, given the wide sampling effort in space and time. Historical fishery time-series, therefore, represent “a vital component of the fishery management process” (Morgan and Burgess, 2005) whose analysis may highlight decreases in populations that could otherwise have gone unnoticed (Casey and Myers, 1998).
The goal of this study was to investigate the past and present status of elasmobranchs in the northern Adriatic Sea in relation to fishing pressure and environmental factors, using fishery data from the fish market of Chioggia, Italy, home of the major fishing fleet of the basin. This goal was achieved by (i) analysing long-term (1945–2012) trends in elasmobranch landings in relation to human pressures (fishing capacity, nutrient inputs), local hydrography (sea water temperature, river inflow), and large-scale climatic indices (North Atlantic Oscillation, Western Mediterranean Oscillation), and (ii) assessing the current composition of elasmobranch catch in terms of species, size, sex, and sexual maturity through surveys at the fish market of Chioggia from 2006–2007 and 2011–2013.
Methods
Study area
The northern Adriatic Sea is a shallow (29 m average depth) Mediterranean subbasin of ∼32 000 km2 that is all but landlocked by Croatia, Italy and Slovenia (Figure 1). Physical and biological characteristics make the northern Adriatic Sea a peculiar ecosystem, distinct from the rest of the Adriatic. It presents high, but variable, primary productivity, supported by the large nutrient loads mainly discharged by the Italian rivers, particularly the Po River. Temperature, salinity and circulation show marked spatial and temporal (interseasonal, interannual and decadal) variations driven by atmospheric forces and river discharges (Giani et al., 2012).
The Adriatic Sea. The location of Chioggia (circle) and the fishing ground targeted by its fleet in the northern Adriatic Sea (shaded area) are indicated.
Fishery landings data
Official landings data from Chioggia's fishery fleet were retrieved from the Clodia database (Clodia database, 2013). Data (live weight in kilograms) were available yearly from 1945–2012. From 1945–1996, elasmobranchs were grouped into three main categories: sharks (mainly including Mustelus spp. and Squalus spp.), skates (Raja spp.), and catsharks (Scyliorhinus spp.); from 1997, elasmobranchs were recorded in nine categories, according to genus (Table 1). Additionally, Alopias vulpinus was occasionally reported separately before 1997; in such cases, its landings were added to the sharks category. The Myliobatis aquila catch was added to the Raja spp. data because it was occasionally reported individually, often in negligible amounts. At times, a category of unidentified skinned sharks was recorded, which was added to the sharks (1945–1996) or smooth-hounds category (1997–2012).
Categories of elasmobranchs registered at the Chioggia fish market during the two periods: 1945–1996 and 1997–2012.
| Species . | Categories 1945–1996 . | Categories 1997–2012 . |
|---|---|---|
| Alopias vulpinus | Sharks | Thresher |
| Carcharhinus plumbeus | Sharks | Sandbar shark |
| Galeorhinus galeus | Sharks | Tope shark |
| Lamna nasus | Sharks | Porbeagle |
| Myliobatis aquila | Skates | Skates |
| Mustelus spp. | Sharks | Smooth-hounds |
| Prionace glauca | Sharks | Blue shark |
| Raja spp. | Skates | Skates |
| Scyliorhinus spp. | Catsharks | Catsharks |
| Squalus spp. | Sharks | Dogfish |
| Species . | Categories 1945–1996 . | Categories 1997–2012 . |
|---|---|---|
| Alopias vulpinus | Sharks | Thresher |
| Carcharhinus plumbeus | Sharks | Sandbar shark |
| Galeorhinus galeus | Sharks | Tope shark |
| Lamna nasus | Sharks | Porbeagle |
| Myliobatis aquila | Skates | Skates |
| Mustelus spp. | Sharks | Smooth-hounds |
| Prionace glauca | Sharks | Blue shark |
| Raja spp. | Skates | Skates |
| Scyliorhinus spp. | Catsharks | Catsharks |
| Squalus spp. | Sharks | Dogfish |
The species list has been reconstructed according to the species recorded at the fish market during the surveys.
Categories of elasmobranchs registered at the Chioggia fish market during the two periods: 1945–1996 and 1997–2012.
| Species . | Categories 1945–1996 . | Categories 1997–2012 . |
|---|---|---|
| Alopias vulpinus | Sharks | Thresher |
| Carcharhinus plumbeus | Sharks | Sandbar shark |
| Galeorhinus galeus | Sharks | Tope shark |
| Lamna nasus | Sharks | Porbeagle |
| Myliobatis aquila | Skates | Skates |
| Mustelus spp. | Sharks | Smooth-hounds |
| Prionace glauca | Sharks | Blue shark |
| Raja spp. | Skates | Skates |
| Scyliorhinus spp. | Catsharks | Catsharks |
| Squalus spp. | Sharks | Dogfish |
| Species . | Categories 1945–1996 . | Categories 1997–2012 . |
|---|---|---|
| Alopias vulpinus | Sharks | Thresher |
| Carcharhinus plumbeus | Sharks | Sandbar shark |
| Galeorhinus galeus | Sharks | Tope shark |
| Lamna nasus | Sharks | Porbeagle |
| Myliobatis aquila | Skates | Skates |
| Mustelus spp. | Sharks | Smooth-hounds |
| Prionace glauca | Sharks | Blue shark |
| Raja spp. | Skates | Skates |
| Scyliorhinus spp. | Catsharks | Catsharks |
| Squalus spp. | Sharks | Dogfish |
The species list has been reconstructed according to the species recorded at the fish market during the surveys.
Fishing capacity and hydroclimatic time-series
The Chioggia fleet is the most important in the northern Adriatic Sea, fishing mainly in the northern part of the Adriatic (Barausse et al., 2011). The fishing vessels are equipped with one or more types of fishing gear (e.g. hydraulic dredges, midwater trawls, otter trawls, and beam trawls) or with artisanal fishing equipment such as traps and gillnets. Total fishing capacity was used as a proxy for fishing pressure in the analyses because no long-term records of fishing effort are available. Total fishing capacity was expressed as gross registered tonnage (GRT), from 1951–1992, while as gross tonnage (GT) from 1991–2012 (see Barausse et al., 2011; Clodia database, 2013). Since the two measures were very similar in the overlapping years, we indicated the fishing capacity as GT in the figures and analyses. Using fish market statistics and fishing cooperatives’ data, landings of elasmobranchs in 2007 were attributed to the different fishing gears to estimate their contribution to the catch of each category.
Landings were analysed in relation to several hydroclimatic time-series. The annual load of phosphates (t of P-PO4) discharged by the Po River from 1968–2007, with a gap in 1976 (UNEP/MAP/MEDPOL, 2003; Cozzi and Giani, 2011), was included to represent the effects of past anthropogenic nutrient enrichment and the current oligotrophication of the basin, where phosphorus is the limiting nutrient (Artioli et al., 2008; Barausse et al., 2011). The variability in local hydrography was represented using long-term records (1945–2011; annual averages) of seawater temperature in Trieste (°C) at 2 m depth (Stravisi, 2009; Stravisi and Cirilli, 2012), and of the Po River discharge (m3 s−1) at Pontelagoscuro, near the delta (Barausse et al., 2011, data were integrated using official statistics of the Environmental Protection Agency of the Emilia Romagna Region, http://www.arpa.emr.it/sim/?idrologia/annali_idrologici, last accessed 29 April 2013). Two indices of large-scale climatic oscillations known to influence marine ecosystems (see Barausse et al., 2011 for details) were included: the North Atlantic Oscillation index (NAO, annual mean, 1945–2012; data retrieved from http://www.cru.uea.ac.uk/cru/data/nao/, last accessed 11 June 2013, and http://www.cru.uea.ac.uk/~timo/datapages/naoi.htm, last accessed 11 June 2013) and the winter (December–February) Western Mediterranean Oscillation index (WeMO, 1945–2011; Martin-Vide and Lopez-Bustins, 2006; data 2001–2011 provided by J. A. Lopez-Bustins).
Fish market surveys
Surveys at the fish market were performed from October 2006–July 2007 and from January 2011–April 2013 (missing data: January 2012). The fish market was visited either weekly or biweekly for a total of 250 surveys during either the night- or day-selling auctions, when fish are sold at the wholesale fish market.
At each survey, landed elasmobranchs were counted and identified to the species level according to external morphological characters (Whitehead et al., 1986; Serena, 2005; Serena et al., 2010), with the exception of Mustelus mustelus and M. punctulatus, which were pooled together as Mustelus spp. because of the lack of unambiguous morphological traits for their identification (I. Marino and C. Mazzoldi, unpublished data; see also Discussion regarding Raja asterias and R. clavata identification). All elasmobranchs or a random subsample (in a few cases of very abundant landings) were measured (total length from the tip of the snout to the end of the caudal fin) to the nearest 5 mm with a measuring tape. Sex was attributed based on the presence of claspers in the pelvic fins of males. Because specimens are eviscerated before landing, sexual maturity was directly attributed only to males, based on the length and calcification of claspers (Conrath, 2005), and the presence of sperm in the seminal vesicles, which are usually not removed. Sexual maturity was indirectly attributed to females, using literature estimates of TL50 (the total length at which 50% of females are sexually mature) from the Adriatic Sea (if available) or from the Black or Mediterranean seas (Mustelus spp.: C. Mazzoldi and E. Riginella, unpublished data; Squalus acanthias: Gračan et al., 2013; Scyliorhinus canicula: Kousteni et al., 2010; S. stellaris: Notarbartolo di Sciara and Bianchi, 1998; R. asterias: Romanelli et al., 2007; R. clavata: Saglam and Ak, 2012).
Data analyses
All data are reported as means ± standard deviations. Parametric or non-parametric tests were applied according to data distribution and test assumptions. The Mann–Kendall test was employed to detect temporal trends in the annual landing data categories or other time-series, and, if statistically significant, slopes were computed with Sen's robust estimator (Gilbert, 1987). Given the inconsistency in aggregating species into landing categories, a first analysis was performed on the entire dataset, applying the broad categories used up to 1996 to the more recent data as well. A second analysis, limited to the data from 1997–2012, was applied to three categories that changed after 1996 and contained records for each year: dogfish, smooth-hounds, and thresher (see Table 1). Because of problems caused by underreporting (see Results), data from the sharks category from 1986–1993 were excluded from the trend analysis; consequently, total elasmobranch landings from 1986–1993 were also excluded. However, substituting missing data with a linear interpolation from 1985–1994 did not change the results. Trend analyses were repeated after dividing landings by the fishing capacity (1951–2012) in Chioggia, thus obtaining a simple catch-per-unit-effort (cpue) index, an indicator of the relative abundance of marine resources (Jensen et al., 2012).
The Continuous Wavelet Transform (CWT) was used to investigate fluctuations in the time-series of skates and catsharks from 1945–2012, and of sharks and total elasmobranchs, analysed only up to 1985. Wavelet analysis (Grinsted et al., 2004) highlights the dominant periodicities in a time-series, which correspond to its strongest oscillations, indicating whether such oscillations are evenly distributed over time or concentrated in particular periods (e.g. intermittent or isolated peaks). Time-series were percentile-transformed and analysed using the Morlet wavelet; significance levels were assessed based on the null hypothesis of a first-order autoregressive process, i.e. red noise background (Grinsted et al. 2004). Computations were run using A. Grinsted's MATLAB® code (http://noc.ac.uk/using-science/crosswavelet-wavelet-coherence, last accessed 13 June 2013).
Correlations were tested between the annual landings time-series of the three elasmobranch categories over 1945–2012 and fishing capacity (to investigate whether changes in landings reflect variations in the size of the fishing fleet), and between cpue and hydroclimatic time-series (to study the influence of environmental variability on population dynamics), using Spearman's rank correlation coefficient, chosen because some of the time-series could not be normalized. The Benjamini–Hochberg FDR procedure was followed to correct for multiple testing (Verhoeven et al., 2005). Predictive variables were not excessively multicollinear (for all correlations between variables, Spearman's rs ≤ 0.5; Anderson et al., 2008). Correlations between cpue and hydroclimatic time-series were also calculated using a time-lag based on the dominant periodicity of the landing categories, which was drawn from the wavelet analyses.
In the data collected at the fish market, the sex ratio was calculated for each sampling period (October 2006–July 2007, January–December 2011; February 2012–April 2013) for species presenting sample size >80 in each sampling period. Differences from an expected sex ratio of 1:1 were checked with the Chi-square test.
Results
Fishery landings data
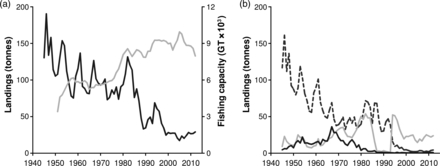
Elasmobranch landings ranged from 5.4% (1945) to 0.2% (2007) of the total landings of the Chioggia fleet. From 1945–2012, total landings of elasmobranchs continuously declined (Figure 2a; Table 2), with catches during the last five years representing 18.6% of those in the 1940s. However, fishing capacity increased from 1951–2012 (Mann–Kendall Z = 7.82, p < 0.0001, Sen's slope = +81.6 GT year−1; Figure 2a); therefore, cpue decreased (Table 2).
Trends in the annual time-series of elasmobranch categories.
| . | Landings . | Cpue . | ||
|---|---|---|---|---|
| Category . | Mann–Kendall test . | Sen's slope . | Mann–Kendall test . | Sen's slope . |
| Categories recorded since 1945 | ||||
| Total elasmobranchs | Z = −7.19, p = 1.8 × 10−12* | −1674 kg year−1 | Z = −7.69, p = 5.1 × 10−14* | −0.361 kg GT−1 year−1 |
| Skates | Z = −8.82, p = 8.1 × 10−18* | −1519 kg year−1 | Z = −9.13, p = 9.6 × 10−19* | −0.254 kg GT−1 year−1 |
| Catsharks | Z = −6.60, p = 9.9 × 10−11* | −248 kg year−1 | Z = −7.81, p = 2.8 × 10−14* | −0.061 kg GT−1 year−1 |
| Sharks | Z = 3.50, p = 0.0009* | +228 kg year−1 | Z = −0.56, p = 0.62 | − |
| Categories recorded since 1997 | ||||
| Thresher | Z = 0.90, p = 0.52 | – | Z = 0.99, p = 0.50 | − |
| Smooth-hounds | Z = −0.63, p = 0.62 | – | Z = −0.09, p = 0.93 | − |
| Dogfish | Z = −0.99, p = 0.50 | – | Z = −0.72, p = 0.60 | − |
| . | Landings . | Cpue . | ||
|---|---|---|---|---|
| Category . | Mann–Kendall test . | Sen's slope . | Mann–Kendall test . | Sen's slope . |
| Categories recorded since 1945 | ||||
| Total elasmobranchs | Z = −7.19, p = 1.8 × 10−12* | −1674 kg year−1 | Z = −7.69, p = 5.1 × 10−14* | −0.361 kg GT−1 year−1 |
| Skates | Z = −8.82, p = 8.1 × 10−18* | −1519 kg year−1 | Z = −9.13, p = 9.6 × 10−19* | −0.254 kg GT−1 year−1 |
| Catsharks | Z = −6.60, p = 9.9 × 10−11* | −248 kg year−1 | Z = −7.81, p = 2.8 × 10−14* | −0.061 kg GT−1 year−1 |
| Sharks | Z = 3.50, p = 0.0009* | +228 kg year−1 | Z = −0.56, p = 0.62 | − |
| Categories recorded since 1997 | ||||
| Thresher | Z = 0.90, p = 0.52 | – | Z = 0.99, p = 0.50 | − |
| Smooth-hounds | Z = −0.63, p = 0.62 | – | Z = −0.09, p = 0.93 | − |
| Dogfish | Z = −0.99, p = 0.50 | – | Z = −0.72, p = 0.60 | − |
The table reports the Z statistic and associated p-value (adjusted for 14 multiple tests) of the Mann–Kendall test for temporal trends in landing or cpue time-series of elasmobranchs; for statistically significant trends at the 0.05 level (highlighted by asterisk), Sen's robust estimate of the rate of change of the time-series is given (Gilbert, 1987). For total elasmobranchs and sharks, the years 1986–1993 were excluded from the calculations. The cpue indices of the elasmobranch categories recorded from 1945 could only be calculated from 1951.
Trends in the annual time-series of elasmobranch categories.
| . | Landings . | Cpue . | ||
|---|---|---|---|---|
| Category . | Mann–Kendall test . | Sen's slope . | Mann–Kendall test . | Sen's slope . |
| Categories recorded since 1945 | ||||
| Total elasmobranchs | Z = −7.19, p = 1.8 × 10−12* | −1674 kg year−1 | Z = −7.69, p = 5.1 × 10−14* | −0.361 kg GT−1 year−1 |
| Skates | Z = −8.82, p = 8.1 × 10−18* | −1519 kg year−1 | Z = −9.13, p = 9.6 × 10−19* | −0.254 kg GT−1 year−1 |
| Catsharks | Z = −6.60, p = 9.9 × 10−11* | −248 kg year−1 | Z = −7.81, p = 2.8 × 10−14* | −0.061 kg GT−1 year−1 |
| Sharks | Z = 3.50, p = 0.0009* | +228 kg year−1 | Z = −0.56, p = 0.62 | − |
| Categories recorded since 1997 | ||||
| Thresher | Z = 0.90, p = 0.52 | – | Z = 0.99, p = 0.50 | − |
| Smooth-hounds | Z = −0.63, p = 0.62 | – | Z = −0.09, p = 0.93 | − |
| Dogfish | Z = −0.99, p = 0.50 | – | Z = −0.72, p = 0.60 | − |
| . | Landings . | Cpue . | ||
|---|---|---|---|---|
| Category . | Mann–Kendall test . | Sen's slope . | Mann–Kendall test . | Sen's slope . |
| Categories recorded since 1945 | ||||
| Total elasmobranchs | Z = −7.19, p = 1.8 × 10−12* | −1674 kg year−1 | Z = −7.69, p = 5.1 × 10−14* | −0.361 kg GT−1 year−1 |
| Skates | Z = −8.82, p = 8.1 × 10−18* | −1519 kg year−1 | Z = −9.13, p = 9.6 × 10−19* | −0.254 kg GT−1 year−1 |
| Catsharks | Z = −6.60, p = 9.9 × 10−11* | −248 kg year−1 | Z = −7.81, p = 2.8 × 10−14* | −0.061 kg GT−1 year−1 |
| Sharks | Z = 3.50, p = 0.0009* | +228 kg year−1 | Z = −0.56, p = 0.62 | − |
| Categories recorded since 1997 | ||||
| Thresher | Z = 0.90, p = 0.52 | – | Z = 0.99, p = 0.50 | − |
| Smooth-hounds | Z = −0.63, p = 0.62 | – | Z = −0.09, p = 0.93 | − |
| Dogfish | Z = −0.99, p = 0.50 | – | Z = −0.72, p = 0.60 | − |
The table reports the Z statistic and associated p-value (adjusted for 14 multiple tests) of the Mann–Kendall test for temporal trends in landing or cpue time-series of elasmobranchs; for statistically significant trends at the 0.05 level (highlighted by asterisk), Sen's robust estimate of the rate of change of the time-series is given (Gilbert, 1987). For total elasmobranchs and sharks, the years 1986–1993 were excluded from the calculations. The cpue indices of the elasmobranch categories recorded from 1945 could only be calculated from 1951.
Annual fishery time-series in Chioggia. (a) Total elasmobranch landings at the fish market of Chioggia, 1945–2012 (black line, left axis), and fishing capacity (GT × 103) of the Chioggia fishing fleet, 1951–2012 (grey line, right axis). (b) Landings of the three categories comprising the elasmobranch landings in 1945–2012: skates (dashed black line), catsharks (continuous black line), sharks (continuous grey line).
The analysis of the three categories (sharks, catsharks and skates; Figure 2b) highlighted striking changes in landings composition from a predominance of skates in the 1940s (85.0%) to a predominance of sharks during 2008–2012 (86.2%).
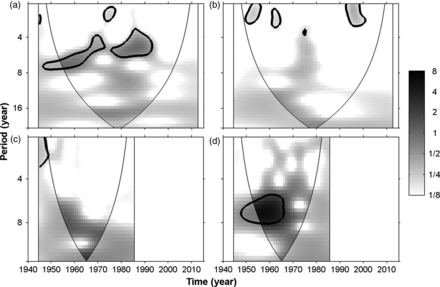
Skates experienced the most dramatic reduction, both in landings and in cpue (Figure 2b; Table 2). Landings during 2008–2012 represented only 2.4% of their value in 1945–1949. Since 1994, landings collapsed nearly to zero (4966 kg year−1 on average). Strong fluctuations in landings were present during the 1940s and 1950s, displaying a significant periodicity of ∼7 years; the fluctuations became more frequent over time, reaching a periodicity of ∼4–6 years from the late 1960s onwards, and they disappeared after 1994, following the collapse of the landings (Figure 3a).
Continuous wavelet power spectrum of the landings of elasmobranch categories. (a) skates; (b) catsharks; (c) sharks; (d) total elasmobranchs. The wavelet power is represented in normalized variance units. The 0.05 significance level is given by the thick contours, while the areas where results are potentially biased by the proximity to the start or to the end of the time-series are indicated by the paler shades outside the so-called cone of influence.
Landings of catsharks increased until 1967 and then began to decline, collapsing in 1987 and never recovering to previous levels (Figure 2b). Overall, their landings strongly declined from 1945–2012, similarly to cpue (Table 2). Recent landings (2008–2012) were only 10.6% of those in the 1940s. Wavelet analysis highlighted no dominant fluctuations over the analysed period, apart from a few isolated bursts with a periodicity of 2–3 years (Figure 3b).
Unlike other categories, the landings of sharks increased significantly and, during the last five years, reached 160.7% of the landings recorded in the 1940s. However, when analysing cpue, a corresponding positive trend was not detected (Figure 2b; Table 2). The trajectory of this category over time was quite variable: shark landings increased from 1945–1985, abruptly decreased to near 0 from 1986–1993, and suddenly returned to the level of the early 1980s in 1994. Finally, a strong decline took place from 1994–1999, followed by a phase of relatively constant landings. The 1986–1993 collapse was related to a law introduced in those years requiring the analysis of mercury concentration in shark meat, for which concentrations could not exceed 0.7 ppm. In 1992, the law was changed, requiring the analysis of mercury concentrations only in large sharks and raising the acceptable threshold to 1 ppm. As a consequence, from 1986–1993, sharks were mostly sold illegally, while after 1993, they were again registered in the fish market statistics (Clodia database, 2013). No significant fluctuations were detected by the CWT analysis during 1945–1985, although three peaks and troughs in landings every 6–8 years are present until the early 1960s (Figures 2b and 3c).
Fishing capacity was not correlated with the landings of sharks (rs = 0.24, adjusted p = 0.08), but it was strongly and negatively correlated with catshark (rs = –0.90, adjusted p = 1.9 × 10−23) and skate (rs = –0.71, adjusted p = 1.1 × 10−10) landings, even when the years 1987–2012 and 1994–2012, respectively, were excluded to assess whether the correlation was due to the final landings collapse.
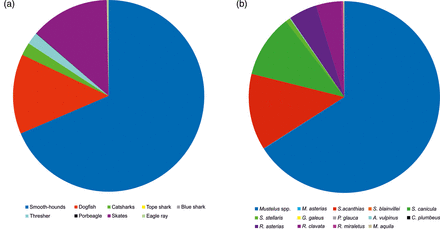
The analysis of landings records during 1997–2012 highlighted that the Mustelus spp. category represented, on average, 68.8% of the landed elasmobranchs; Squalus spp., 13.4%; skates, 13.1%; Scyliorhinus spp., 2.2%; and A. vulpinus 2.1%. All other elasmobranchs together contributed < 0.4% of the total landings (Figure 4a). Landings or cpue exhibited no trend (Table 2).
Composition of elasmobranch landings. (a) official fish market statistics (1997–2012; biomass data); (b) surveys at the fish market (number of individuals).
All the hydroclimatic time-series except river flow (Mann–Kendall Z = 1.22, adjusted p = 0.22) displayed statistically significant trends over time: temperature increased (Z = 3.60, adjusted p = 0.0009, slope = +0.01°C year−1), while phosphate load (Z = –2.24, adjusted p = 0.04, slope = –74.1 t of P-PO4 year−1), WeMO (Z = –3.57, adjusted p = 0.0009, slope = –0.015 year−1) and NAO (Z = –2.16, adjusted p = 0.04, slope = –0.008 year−1) decreased.
Each of the three main categories of elasmobranch cpue displayed significant correlations with the hydroclimatic time-series over the past decades (Table 3; Supplementary material: Figures S1–S6). Skates correlated positively with WeMO and phosphate load and negatively with temperature; catsharks displayed a negative relationship with temperature and a positive relationship with WeMO; sharks were positively correlated with phosphate load. Lagged correlations (3 years: catsharks; 6 years: skates and sharks) gave similar results; therefore, they are not shown. When the years in which landings collapsed for catsharks (1987–2012) and skates (1994–2012) were excluded from the analysis, all previously significant correlations disappeared (p ≥ 0.08 after adjusting for multiple tests) except skates – WeMO (rs = 0.48, adjusted p = 0.016), and a negative correlation emerged between catsharks and phosphate load (rs = –0.67, adjusted p = 0.016).
Correlations between cpue of elasmobranch categories (1945–2012) and hydroclimatic time-series.
| Hydroclimatic time-series . | Cpue categories (1945–2012) . | ||
|---|---|---|---|
| Sharks . | Catsharks . | Skates . | |
| Po River flow | rs = 0.17, n = 53, p = 0.32 | rs = 0.06, n = 61, p = 0.71 | rs = 0.00, n = 61, p = 0.99 |
| Water temperature | rs = –0.20, n = 53, p = 0.22 | rs = –0.44, n = 61, p = 0.003* | rs = –0.41, n = 61, p = 0.006* |
| Phosphate load | rs = 0.43, n = 31, p = 0.04* | rs = 0.29, n = 39, p = 0.12 | rs = 0.49, n = 39, p = 0.006* |
| NAO | rs = 0.08, n = 54, p = 0.63 | rs = 0.10, n = 62, p = 0.54 | rs = 0.24, n = 62, p = 0.11 |
| WeMO | rs = 0.26, n = 53, p = 0.11 | rs = 0.32, n = 61, p = 0.03* | rs = 0.50, n = 61, p = 0.0006* |
| Hydroclimatic time-series . | Cpue categories (1945–2012) . | ||
|---|---|---|---|
| Sharks . | Catsharks . | Skates . | |
| Po River flow | rs = 0.17, n = 53, p = 0.32 | rs = 0.06, n = 61, p = 0.71 | rs = 0.00, n = 61, p = 0.99 |
| Water temperature | rs = –0.20, n = 53, p = 0.22 | rs = –0.44, n = 61, p = 0.003* | rs = –0.41, n = 61, p = 0.006* |
| Phosphate load | rs = 0.43, n = 31, p = 0.04* | rs = 0.29, n = 39, p = 0.12 | rs = 0.49, n = 39, p = 0.006* |
| NAO | rs = 0.08, n = 54, p = 0.63 | rs = 0.10, n = 62, p = 0.54 | rs = 0.24, n = 62, p = 0.11 |
| WeMO | rs = 0.26, n = 53, p = 0.11 | rs = 0.32, n = 61, p = 0.03* | rs = 0.50, n = 61, p = 0.0006* |
Spearman's coefficient (rs) is reported along with sample size (n) and the adjusted p-value for multiple testing. Asterisks highlight statistically significant correlations at the 0.05 level. Sharks data for 1986–1993 were excluded from the analyses (see Results).
Correlations between cpue of elasmobranch categories (1945–2012) and hydroclimatic time-series.
| Hydroclimatic time-series . | Cpue categories (1945–2012) . | ||
|---|---|---|---|
| Sharks . | Catsharks . | Skates . | |
| Po River flow | rs = 0.17, n = 53, p = 0.32 | rs = 0.06, n = 61, p = 0.71 | rs = 0.00, n = 61, p = 0.99 |
| Water temperature | rs = –0.20, n = 53, p = 0.22 | rs = –0.44, n = 61, p = 0.003* | rs = –0.41, n = 61, p = 0.006* |
| Phosphate load | rs = 0.43, n = 31, p = 0.04* | rs = 0.29, n = 39, p = 0.12 | rs = 0.49, n = 39, p = 0.006* |
| NAO | rs = 0.08, n = 54, p = 0.63 | rs = 0.10, n = 62, p = 0.54 | rs = 0.24, n = 62, p = 0.11 |
| WeMO | rs = 0.26, n = 53, p = 0.11 | rs = 0.32, n = 61, p = 0.03* | rs = 0.50, n = 61, p = 0.0006* |
| Hydroclimatic time-series . | Cpue categories (1945–2012) . | ||
|---|---|---|---|
| Sharks . | Catsharks . | Skates . | |
| Po River flow | rs = 0.17, n = 53, p = 0.32 | rs = 0.06, n = 61, p = 0.71 | rs = 0.00, n = 61, p = 0.99 |
| Water temperature | rs = –0.20, n = 53, p = 0.22 | rs = –0.44, n = 61, p = 0.003* | rs = –0.41, n = 61, p = 0.006* |
| Phosphate load | rs = 0.43, n = 31, p = 0.04* | rs = 0.29, n = 39, p = 0.12 | rs = 0.49, n = 39, p = 0.006* |
| NAO | rs = 0.08, n = 54, p = 0.63 | rs = 0.10, n = 62, p = 0.54 | rs = 0.24, n = 62, p = 0.11 |
| WeMO | rs = 0.26, n = 53, p = 0.11 | rs = 0.32, n = 61, p = 0.03* | rs = 0.50, n = 61, p = 0.0006* |
Spearman's coefficient (rs) is reported along with sample size (n) and the adjusted p-value for multiple testing. Asterisks highlight statistically significant correlations at the 0.05 level. Sharks data for 1986–1993 were excluded from the analyses (see Results).
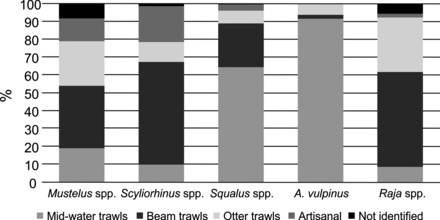
In 2007, most elasmobranchs were landed as bycatch (89.1 ± 9.9%, range: 78.4–100%): Mustelus spp., Scyliorhinus spp., and skates mainly as the bycatch of otter and beam trawls, and Squalus spp. and A. vulpinus mainly of midwater trawling. The artisanal fishery, periodically targeting elasmobranchs, contributed little to the total catch (Figure 5).
Fish market surveys
A total of 11 900 specimens, belonging to 14 species, were sampled. In all sampling years, the landings of elasmobranch were dominated by Mustelus spp., representing from 61.4–68.8% of the total (Figure 4b). Very few individuals of the large-sized species, Prionace glauca, A. vulpinus, Galeorhinus galeus and Carcharhinus plumbeus, were recorded. Additionally, the smaller-sized M. asterias and S. blainville were sampled with six and one specimens, respectively, in the 2006–2007 surveys. The comparison of landings composition based on numbers (average data of fish market surveys from all the sampling years) and based on biomass (average landings data for 1997–2012) indicates that the two methods produce a similar picture, even if fish market statistics tend to underestimate the small-sized catsharks and overestimate skates and threshers (Figure 4a and b).
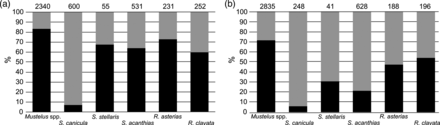
The percentage of immature individuals landed at the fish market was high for most of the analysed species except S. canicula (Figure 6a and b). In particular, it was > 60% for females of Mustels spp., S. acanthias, S. stellaris, R. asterias and R. clavata. R. asterias presented 13 individuals (3.6% of the total) with sizes larger than the maximum reported in the literature.
Sexual maturity of landed elasmobranch. (a) females; (b) males. The percentages of immature individuals are represented in black, the mature ones in grey. Numbers above bars represent sample size.
In all sampling years, the sex ratio was significantly skewed towards females in S. canicula (males/total: 0.30 ± 0.04; in all years p < 0.001), while slightly skewed sex ratios were found for the other species that were not consistent among years.
Discussion
Our results highlighted a dramatic decline, of ∼ 80%, in elasmobranch landings over the past 68 years in the northern Adriatic Sea. The decline is more severe after landings are corrected for changes in fishing capacity, which increased over time. The cpue of elasmobranchs declined by 89% in the last 62 years, similar to the 94% decline obtained by comparing scientific surveys performed with demersal trawls in 1948 and 2005 in the Adriatic Sea (Ferretti et al., 2013). Our data extend the depiction by Ferretti et al. (2013): the use of landings data allowed us to comprehensively depict the state of commercially important elasmobranchs in the northern Adriatic Sea, both in time (due to the use of continuous data), and space (considering the extent of fishing grounds covered by the fleet and the use of fishing gear targeting both the water column and the bottom). Although statistics from the fish market can be biased by many factors (Jensen et al., 2012), as highlighted by the unreported landings of sharks from 1986–1993, local knowledge can help clarify whether these issues affect ecological interpretation. In Chioggia, fishermen confirmed that the decrease in landed elasmobranchs reflects a reduction in catches, rather than an increase in underreporting or a decrease in market demand, which are unsupported (C. Mazzoldi, unpublished interviews). Elasmobranchs mainly represent bycatch of the Chioggia fleet; therefore, changes in landings are unlikely to reflect only changes in fishing gear. The high fishing pressure exerted in a small, landlocked area, such as the northern Adriatic Sea, makes it implausible that such a decrease in landings can be attributed to marked changes in fishing grounds, as suggested for other Mediterranean zones (Abella and Serena, 2005). The decrease in landings and cpue, therefore, likely reflects a real biomass decline.
The grouping of several species into broad categories in fishery statistics eliminates the possibility of reconstructing the decline or increase of single species or highlighting opposite trends in species within categories (Dulvy and Reynolds, 2002). In our work, the sharks category, grouping several species belonging to different families, might be the least informative, given that its composition might have changed over time. However, even this category showed a declining trend after 1994. The skates and catsharks categories include fewer species that are similar in size and in biological and ecological characteristics. These categories showed marked decreases of 98 and 97%, respectively, of the cpue with respect to the first half of the 1950s, thus depicting a substantial population decline regardless of species, in line with results of scientific surveys from 1948–2006 in the Adriatic Sea (Krstulović Šifner et al., 2009; Ferretti et al., 2013). The negative correlation between fishing capacity and the landings of skates and catsharks is unexpected, given that a large fleet should fish more, and this result strongly suggests that the long-term decline of these categories and their collapse two to three decades ago is due to overfishing. In general, the pervasive, continuous decline of all elasmobranch categories highlighted by our study is consistent with long-term excessive exploitation, a hypothesis already advanced by other authors for the Adriatic (Fortibuoni et al., 2010; Barausse et al., 2011; Ferretti et al., 2013).
Fish market sampling revealed a current composition of landed elasmobranchs that is strongly dominated by just two species, the smooth-hounds M. mustelus and M. punctulatus, which were pooled together due to uncertainty in their identification, while the remaining species modestly contributed to the sample. A possible misidentification of R. asterias was highlighted by the occurrence of individuals larger than the maximum reported size attributed to this species. Considering the ambiguity in the identification of R. asterias and R. clavata raised by Tinti et al. (2003), these results call for caution when estimating the actual proportions of these two species in the landings when using fish market surveys. Regardless of the possible misidentification of these two pairs of species, the general landings composition appears consistent from 2006–2013 and is in agreement with the image emerging from the landing statistics. In addition to the dominance of a few species, the surveys at the fish market recorded only 15 of the 48 species reported for the northern-central Adriatic Sea (Vacchi and Serena, 2010), most of which were mesopredators (Ferretti et al., 2013). Even if the lack of past landings composition data does not permit detailed analyses, and our surveyed data could be biased by fishery selectivity and, possibly, preferential landings for more valuable species, by comparing these findings with scientific surveys (Ferretti et al., 2013), some long-term changes in the composition of elasmobranch communities in the northern Adriatic can be highlighted. Several species present in the basin in the past were not detected in our surveys, and, indeed, some may have almost disappeared from the study area in recent decades due to overfishing, e.g. the skates Dipturus batis, Rostroraja alba and Raja montagui (Ferretti et al., 2013). Other species could have been rarely or never recorded during the fish market surveys due to their low or null commercial value, e.g. torpedos, sting rays and eagle rays, or, in the case of M. asterias, S. stellaris, and S. blainville, due to low abundance in the study area (Ferretti et al., 2013). The low occurrence of Raja miraletus is in some disagreement with the results of scientific surveys highlighting an increase in this species (Ferretti et al., 2013). Other rarely recorded species include the large-sized mesopredators G. galeus and C. plumbeus and the top predators A. vulpinus, Lamna nasus and P. glauca, considered to be largely depleted even before 1945 in the basin, likely due to the long history of exploitation and human impact on the basin that date back to the first available quantitative data (Ferretti et al., 2008, 2013; Fortibuoni et al., 2010). The overfishing of large predators even before the 1940s, releasing smaller elasmobranchs from predation, might explain the first part of the time-series for catsharks and sharks, whose landings were increasing until the late 1960s and late 1970s, respectively (Ferretti et al., 2013).
Our estimates of the sexual maturity of specimens landed at the fish market further highlighted the unsustainability of the current harvest of elasmobranchs in the Adriatic Sea. Immature specimens represented an astonishing 60–83% of the sampled females and 21–71% of the sampled males, although small-sized individuals are usually discarded at sea (C. Mazzoldi, unpublished data). The smallest-sized species, S. canicula, is the only species presenting a low percentage of immature individuals in the landings (7 and 6%, respectively, for females and males). This result could be related to the discard of small individuals that have negligible commercial value, or to the low catchability of small individuals, possibly due to the occupancy of habitats different from those used by the larger small-spotted catsharks (S. canicula) rather than being directly related to the size of this species, given that catsharks are mainly caught as bycatch by beam trawls, which target small species such as scallop (Pecten jacobaeus), sole (Solea solea) and cuttlefish (Sepia officinalis). The small-spotted catshark is also the only species presenting a skewed sex ratio, with a greater number of females in the catch. Sexual segregation, related to different habitat preferences and/or female avoidance of mature males, has been observed in this species in the Atlantic, also leading to biased sex ratios in the catch (Wearmouth and Sims, 2008).
In addition to general trends in landings and their relation to fishing pressure, our analyses captured two poorly investigated aspects of elasmobranch population dynamics: periodical fluctuations in abundance and their relationship with hydrographic factors and climate. The periodical fluctuations displayed by skate landings likely represent changes in abundance because no fluctuations in fishing capacity were present. Abundance periodicities may reflect population cycles or population outbursts caused by strong year classes, or they may be connected to fluctuations in the abundance of prey or other species, the effects of which propagate across the foodweb. Fluctuations with 5.5–7.3-year and 3–5-year periodicities were observed in zooplankton (Baranovic et al., 1993) and small pelagic fish (Azzali et al., 2002), respectively, in the Adriatic. Fluctuations might also be related to abiotic factors, as suggested by the positive, non-lagged correlation between skate landings and WeMO, an index positively related to the presence of warm, less saline winters in the northern Adriatic (Barausse et al., 2011). Given the slow growth rate and long lifespan of elasmobranchs, a non-lagged response of skates to changes in environmental conditions is likely the result of migrations in and out of the fishing grounds of the Chioggia fleet in relation to climate rather than the outcome of strong year classes. Such migrations could be a direct response to unfavourable environmental conditions, or follow shifts in prey abundance driven by climate. In the Northwest Atlantic, migrations rather than population fluctuations have been advocated as an explanation for winter skate “outbursts” (Frisk et al., 2008). The fluctuations in skate landings increased in frequency as the landings decreased and then disappeared from the time-series when landings collapsed. Fisheries can magnify fluctuations in bony fish abundance (Hsieh et al., 2006), and fishery-induced truncation of the age structure of populations causes unstable population dynamics, reducing the capacity to buffer environmental events (Hsieh et al., 2006). These findings, however, relate to teleost fish, which display higher fecundity and, generally, a lower age of maturity than elasmobranchs, all characteristics that negatively correlate with the coefficient of variation in larval abundance (Hsieh et al., 2006). Conversely, in low-fecundity, late maturing species, such as elasmobranchs, strong fishing pressure might initially magnify fluctuations, but then reduce population abundance to levels so low that they interfere with or mask natural fluctuations, breaking down their relationship with climate oscillations.
Correlation analysis indicated that environmental variability, in addition to fisheries, can influence elasmobranch abundance. In agreement with other scientific observations (Giani et al., 2012), the hydroclimatic time-series show a warming ecosystem, which has recently become less productive because land-based phosphate inputs, peaking from the mid-1970s to the mid-1980s, have since decreased. This ongoing environmental change seems to be detrimental to elasmobranchs. The positive correlation with phosphates suggests that the rise and fall of sharks could be partly related to changes in system productivity; 30–40 years ago, a highly productive ecosystem could sustain a larger number of predators, partly offsetting the effect of fishing pressure, compared with recent years, in which the ecosystem is experiencing oligotrophication (Barausse et al., 2011; Giani et al., 2012). The positive correlation between skates and phosphates could have a similar interpretation. The warming trend of the northern Adriatic basin might have contributed to the decline of catsharks and skates, as indicated by their negative correlation with water temperature. Indeed, in the western Mediterranean, S. canicula tends to prefer deeper, colder waters (Pennino et al., 2013), and catshark landings collapsed in 1987, exactly when the northern Adriatic ecosystem underwent a regime shift connected with an abrupt increase in the warming trend of the northern hemisphere (Conversi et al., 2010; Barausse et al., 2011). Catsharks positively correlated with WeMO, suggesting that warmer and wetter winters may favour, directly or indirectly, these species, possibly influencing their migrations across the boundaries of the ecosystem, as for skates.
One relevant question for conservation and fishery management is whether the influence of the environment and climate on elasmobranch abundance has a comparable effect with that of fishing. The characteristics of the decline of elasmobranchs, i.e. a marked, long-term and multispecies decrease, strongly support the claim that the main cause of the decline is the fishery pressure exerted on the whole basin over many decades. Indeed, a decrease in elasmobranchs was also highlighted before the 1950s by descriptions from naturalists (Fortibuoni et al., 2010). Nutrient enrichment and related eutrophication and anoxic episodes cannot fully explain the long-term elasmobranch decline, as they were limited to relatively short periods, and there is no temporal match between them and changes in elasmobranch abundance. Cultural eutrophication peaked from the mid-1970s to the mid-1980s, well after the decline of skates had started and before the decline of sharks, while anoxic episodes mainly took place during the 1970s and 1980s and rarely affected wide areas of the basin (Artioli et al., 2008; Giani et al., 2012).
What about the role of temperature and climate? When the final years of collapsed catshark and skate landings are removed from the time-series, only two significant correlations remain: (i) a positive relationship between skates and WeMO, discussed above, and (ii) a negative relationship between catsharks and phosphates. This last relationship is difficult to interpret (why should a productive ecosystem be detrimental to elasmobranchs?) and might be spurious. Indeed the sign of this correlation changed from positive to negative when the most recent data were excluded; moreover, this correlation is opposite to that between phosphates and sharks, mainly represented by mesopredators similar to catsharks. The fact that most significant correlations between elasmobranchs and hydroclimatic time-series disappeared when the final years were removed from the analysis suggests that environmental changes were not the chief process driving the continuous, long-term negative trend preceding the collapse of skate and catshark populations. However, unfavourable environmental conditions could have accelerated the final collapse of these categories, acting synergistically with fishing, or prevented a recovery in recent years; hence, they should be accounted for when managing elasmobranch fisheries as a precaution. In conclusion, while the ongoing overfishing of elasmobranchs is clearly illustrated by several results in this work (time-series analysis, fish market records, sexual maturity of market samples), the processes through which the environment influences elasmobranchs are not as clear and deserve further investigation using more sophisticated models.
Conclusions
The results of our study unequivocally show an extraordinary decline in the northern Adriatic elasmobranch fishery, likely reflecting similar declines in population abundance. The most probable cause of such decline appears to be overfishing. This explanation is not new (Fortibuoni et al., 2010; Barausse et al., 2011; Ferretti et al., 2013), but the strength of our work is the comprehensive picture of the state of elasmobranch communities over time, provided by the long-term time-series. Furthermore, our work is not merely descriptive: the long-term dynamics of Adriatic elasmobranchs are discussed for the first time in relation to environmental changes in the ecosystem, and conclusions about overfishing are corroborated by the extensive fish market surveys, which provided clear evidence of growth overfishing. The heavy harvest of immature individuals forecasts a bleak future for elasmobranchs in the northern Adriatic Sea, where the absence of limits in elasmobranch catches likely makes this fishery unsustainable at present levels. Our results indicate that most elasmobranchs are currently landed as bycatch; this finding yields important suggestions for management to improve the conservation status of these fish in the northern Adriatic. High survival rates of discarded elasmobranchs are reported for several species (Revill et al., 2005; Mandelman and Farrington, 2007; Enever et al., 2009); therefore, a management approach that includes minimum size thresholds based on the actual size at maturity of the different species appears promising.
Supplementary data
Funding
The study was supported by the CLODIA project, funded by the Veneto Region (Italy) Law 15/2007 (DGR n. 4069), and the Athenaeum Project CPDA110183, funded by the University of Padova (to CM), and the European Community's Seventh Framework Programme (grant agreement 226675, KnowSeas project) to AB.
Acknowledgements
We wish to thank G. Baldin of the Fish Market of Chioggia and the fishermen for their help with data collection, and F. Badalamenti and M. Rasotto for their revision of a first draft of this paper.
References
Author notes
Handling editor: Emory Anderson